Making CGT Accessible for Medicaid Enrollees: Current Barriers and Proposed Policy Solutions
Thanks to breakthroughs in precision medicine, leading scientists have developed a growing number of cell and gene therapies (CGTs) for serious and life-threatening diseases. By addressing the root causes of disease, these transformative therapies can slow, stop, or even reverse disease progression, achieving life-changing results for patients who previously had no hope of effective treatment.
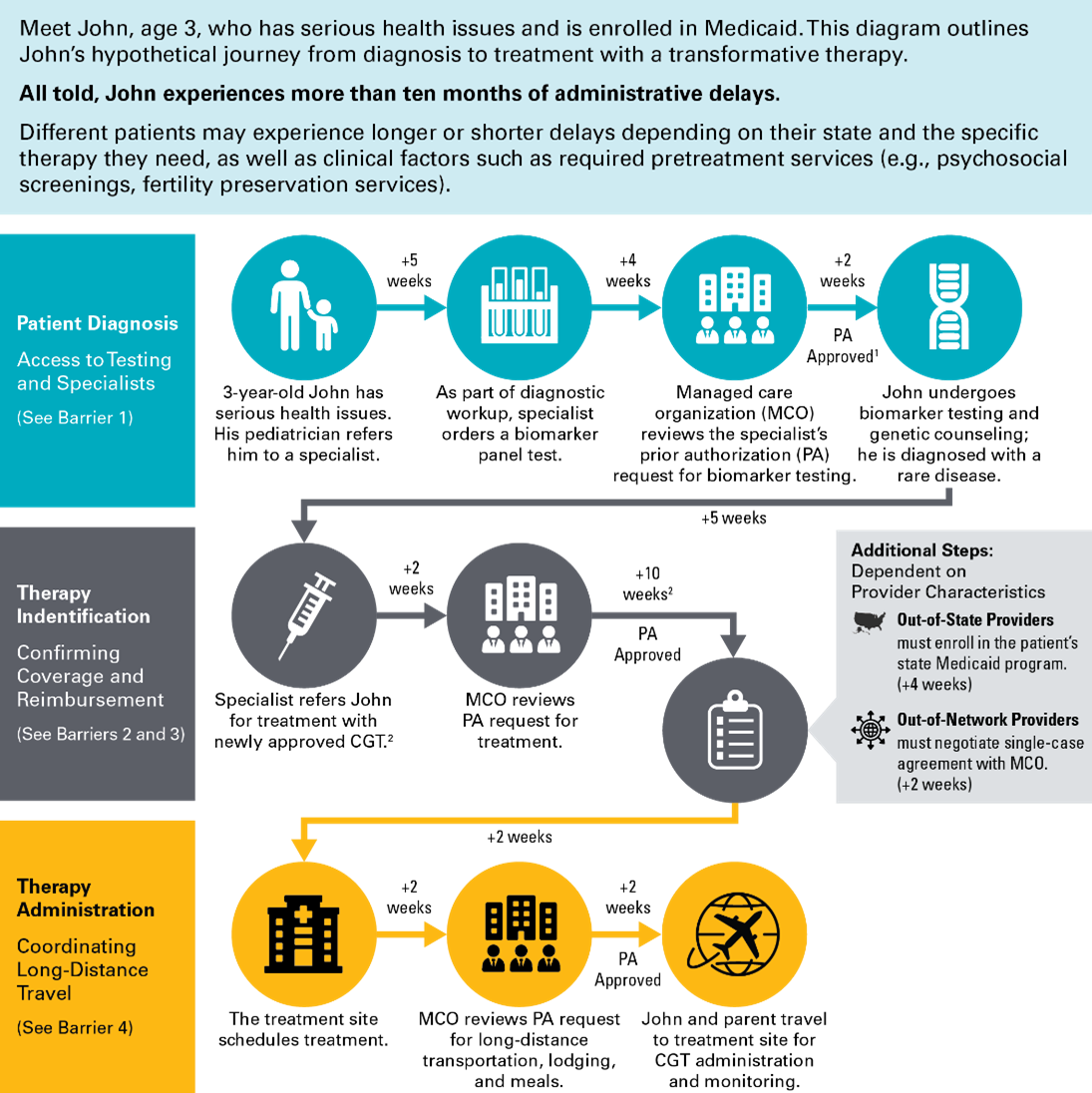
Troublingly, these breakthroughs are not accessible to all. Nationwide, the low-income people covered by the Medicaid program have reduced access to CGTs compared to those covered by Medicare or commercial insurance. On paper, federal law provides that all Medicaid enrollees should have timely access to Food and Drug Administration (FDA)-approved drugs and most specialty care. But in practice, patients and their caregivers can encounter barriers at each step in their journey from diagnosis to treatment.
This paper identifies key barriers that can impede Medicaid patients’ access to CGTs, as well as policy solutions to address those barriers at the state and federal levels. These barriers and solutions were informed by the Manatt CGT Research Collaborative’s survey of relevant Medicaid policies in all 50 states and Washington, D.C., as well as a review of the literature and conversations with diverse stakeholders in the Medicaid ecosystem. To learn more about the Manatt CGT Research Collaborative, email .