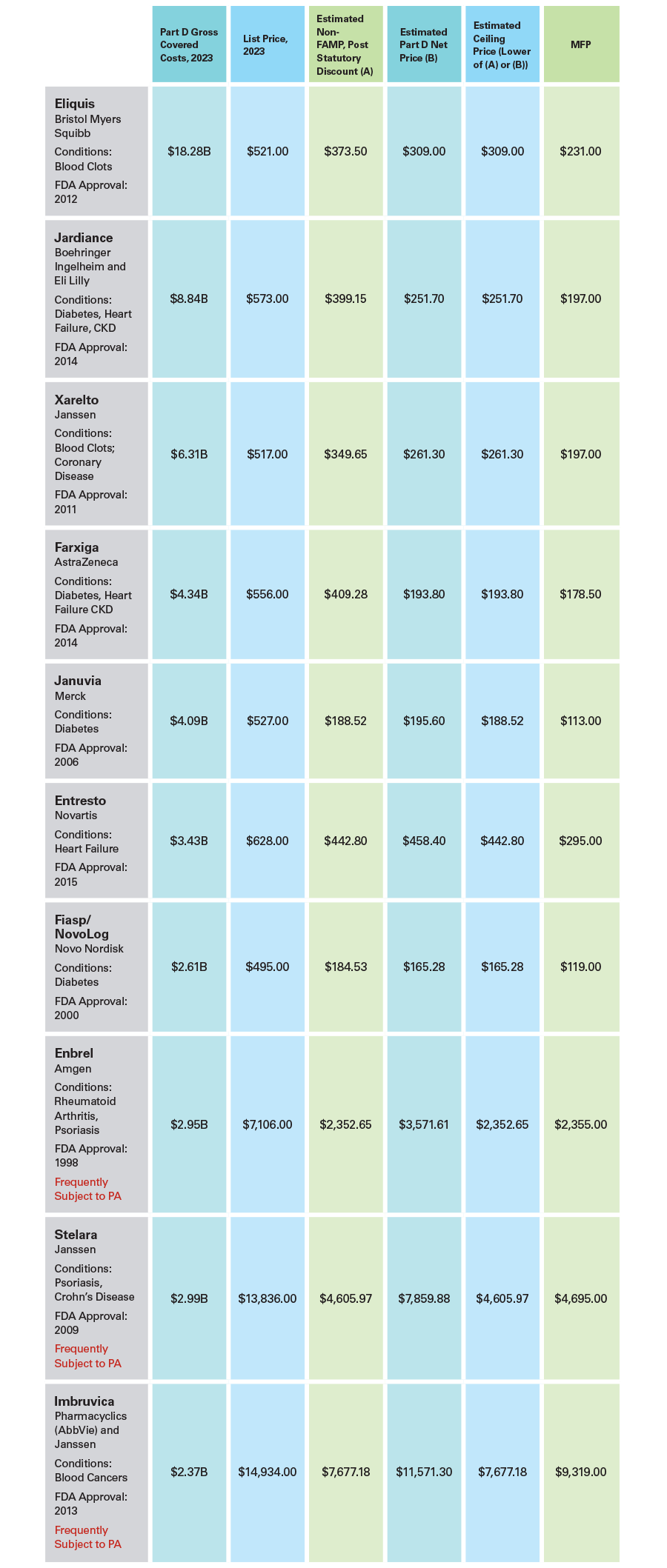
The Inflation Reduction Act Infographic: Overview of Announced Drug Prices
On August 15, 2024, the Centers for Medicare & Medicaid Services (CMS) announced the “maximum fair prices (MFPs)” for the first 10 drugs subject to the Medicare drug negotiation program. Unless the courts or Congress block the program, prices for these drugs under Medicare—as well as under Medicaid and the 340B programs—may not exceed these amounts as of January 1, 2026. CMS has predicted that these prices will save billions for the government, estimating that, had they been in effect for 2023, Medicare would have saved $6 billion in that year alone.
This infographic provides detailed information on the 10 selected drugs and their prices. Under the Inflation Reduction Act (IRA), the MFPs cannot exceed existing Part D net (that is, post-rebate) prices for these drugs, nor can they exceed a percentage of the non-federal average manufacturer price (Non-FAMP). Neither the net prices nor the Non-FAMP have been made public. However, academic researchers have estimated these numbers, and the following compares these estimates to the actual prices dictated by CMS. While the estimates in this infographic are a helpful metric to understanding the Medicare drug negotiation program, they are not perfect. For instance, in some cases, the estimated ceiling price was too low because the estimate fell below the actual MFP, which must be less than or equal to the ceiling price.
It remains to be seen how these prices will impact the Medicare program. Although CMS has observed the prices could lead to lower out-of-pocket cost for some Medicare beneficiaries, other aspects of the IRA—including the $2,000 out-of-pocket cap for annual spending—have a much greater impact on beneficiary cost sharing. Similarly, CMS stated that, had these MFPs been in effect for 2023, net spending for these 10 drug would have been 22% lower. But how much the Medicare program actually will save depends on Part D coverage and utilization of these products in future years, which may change substantially.
Notes: Source for Estimated Non-FAMP, Estimated Part D Net Price, and Estimated Ceiling Price is “Prince Benchmarks of Drugs Selected for Medicare Price Negotiation and Their Therapeutic Alternatives,” J. Managed Care Spec. Pharm. (JMCP), 2024; 30(8):762-772. Manatt did not independently estimate or attempt to verify the accuracy of these figures. The JMCP article presented two sets of prices for Stelara—the higher prices are used here. The JMCP numbers for Novolog/Fiasp were adjusted to reflect an estimated 30-day supply.
Information about which drugs are frequently subject to PA comes from “Medicare Part D Coverage of Drugs Selected for the Drug Price Negotiation Program,” JAMA Health Forum. 2024;5(2).
All figures are for a 30-day supply.